HOME > Information > 2022 > Development of a new staining method for electron microscope specimens --Expected to be a safe, cost-effective, and easy-to-use method--

Information
Development of a new staining method for electron microscope specimens --Expected to be a safe, cost-effective, and easy-to-use method--
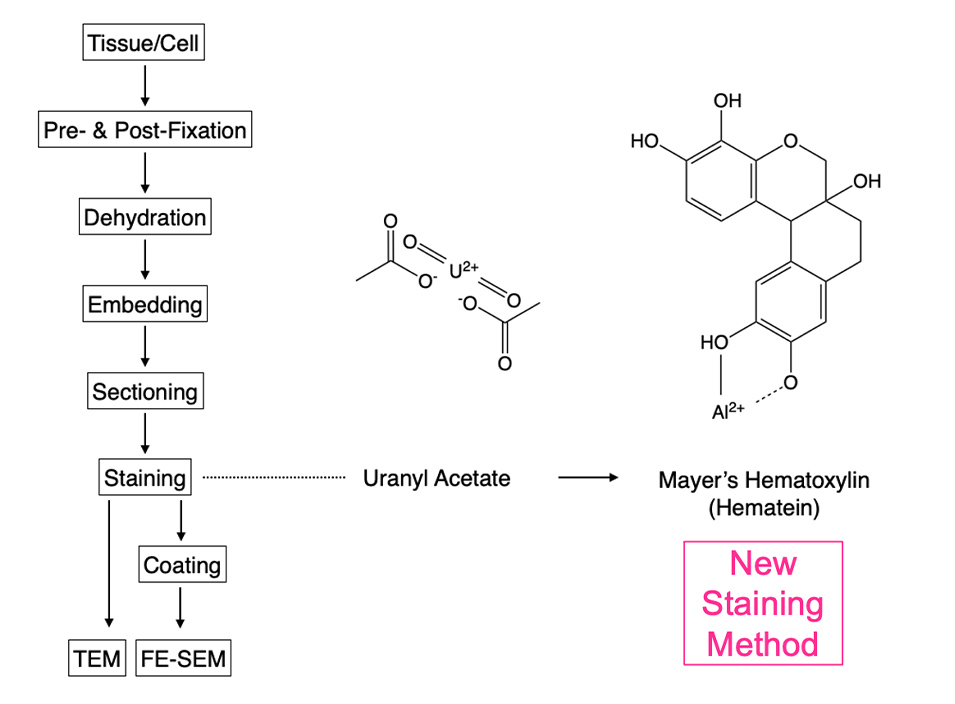
A research group led by Professor Takeshi Matsui (School of Bioscience and Biotechnology, Tokyo University of Technology) and Professor Hiroyuki Sasaki (Department of Rehabilitation Science, Tokyo Professional University of Health Sciences) developed a new staining method for electron microscope specimens. The method is based on double staining with hematoxylin (1), which is widely used as a staining agent for light microscopy, and lead solution. It is expected to replace the conventional method of double staining with uranium acetate and lead solution and is a better choice in terms of safety, cost, and ease of handling.
The research results were published in the online edition of Scientific Reports (2).
Background
The electron microscopic staining technique for biological specimens using uranyl acetate (UA) was first reported in 1958 (3) and has been used in electron microscopy facilities worldwide because of its simplicity and optimal staining results. In recent years, however, international regulations on the use, acquisition, storage, and disposal of uranium compounds have become increasingly strict because uranium is used as a nuclear material for weapons. Against this backdrop, alternative staining methods have long been long awaited in the field of biological research, and several have been proposed, but none has been an effective alternative. In this context, alternative staining methods have long been expected in the bio-medical electron microscopic research field, and several have been proposed, though none has been an effective alternative.
Results
To develop a safe and easy-to-handle alternative staining method to UA for ultrathin sectioning in electron microscopy (4), we examined various commercially available dyes for light microscopy. We found that double staining with Mayer's hematoxylin (MH), which is commonly used as a staining agent in light microscopy, and lead solution (Pb), exhibited staining properties equivalent to those of conventional electron microscopic double staining with UA and Pb in various tissues and cells (Fig. 1).
Other cell organelles, such as nuclear chromatin, plasma membrane structures, ribosomes, glycogen, lipid droplets, cell adhesion apparatus, and cytoskeletal system(s), were stained with high contrast using the MH staining method. Plasma membrane staining in all samples was also satisfactory. (Fig. 1). Backscattered electron images of 200 nm semi-thin sections of mouse kidney observed by a field emission scanning electron microscope also showed that the MH and Pb staining techniques provided a wide-area and high-quality image of the renal cortical tubules and the renal glomerulus (Fig. 1e).
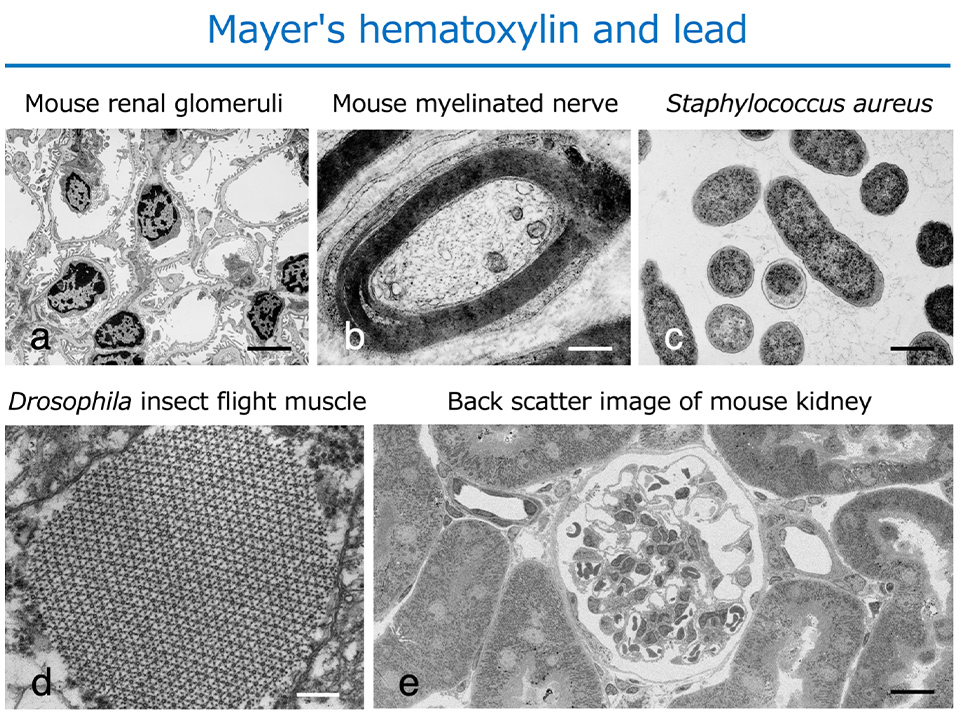
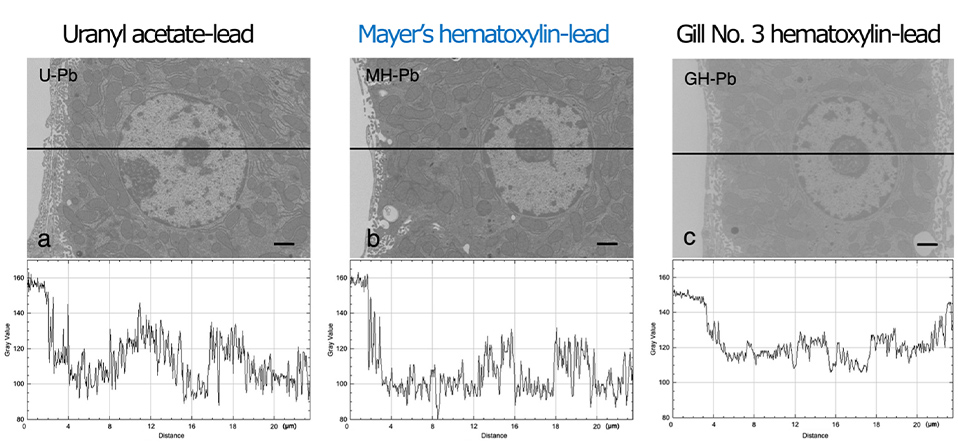
Figure 2 shows the digital images subjected to gray value (i.e., contrast) line analysis to semi-quantitatively determine image quality. The line profile displays two-dimensional graphs of the pixel intensities along the black lines within the images. There was a significant difference in contrast among UA-Pb (Fig. 2a), MH-Pb (Fig. 2b), and Gill No. 3-Pb (Fig.2c), indicating that UA-Pb and MH-Pb enhanced the contrast relative to the cell membrane systems containing cytoplasmic organelles and nuclei. The UA-Pb and MH-Pb line profiles exhibited a greater range of gray values in the cells than the Gill No. 3-Pb line profile.
Social and academic points
MH is a dye solution widely used for staining paraffin sections of diagnostic clinical specimens, and has the advantages of low cost, stable supply as a commercial product, and high safety of waste solution. On the other hand, the International Atomic Energy Agency's "International Basic Safety Standards for Protection against Ionizing Radiation and for the Safety of Radiation Sources" (BSS) has established specific exemption levels, and new regulations for radioactive materials are being developed internationally through legislation. Based on the results of this study, double staining with MH and Pb is expected to become a useful alternative to the staining method using radioactive UA in terms of reagent purchase, handling, use, storage, and liquid waste treatment (Fig. 3).
Glossary
(1) Hematoxylin is a dye extracted from the heartwood of redwood. It is oxidized to hematein, which forms complex salts with aluminum and iron ions to produce a strong blue color.
(2) Sasaki et al., Novel Electron Microscopic Staining Method using Traditional Dye, Hematoxylin. Scientific Reports (2022) (URL: https://www.nature.com/articles/s41598-022-11523-y)
(3) Watson, M. L. Staining of tissue sections for electron microscopy with heavy metals. J. Biophys. Biochem. Cytol. 4(4), 475–478(1958). doi: 10.1083/jcb.4.4.475, Pubmed:13563554
(4) For analysis by transmission electron microscopy, it is necessary to prepare thin films (sections) of approximately 80-100 nm of the resin-embedded sample.
Takeshi Matsui Laboratory (Laboratory for Evolutionary Cell Biology of the Skin) School of Bioscience and Biotechnology, Tokyo University of Technology
We attempted to elucidate the mechanism of stratum corneum barrier formation in the skin epidermis from the perspective of evolutionary biology in terrestrial vertebrates by combining optical and electron microscopy techniques. Research projects:1) Formation mechanism of the skin epidermal stratum corneum barrier; 2) Live imaging of the skin epidermis; 3) Comparative evolutionary cell biology of stratum granulosum cells
Homepage URL: https://takeshi-matsui-lab.bs.teu.ac.jp/?page_id=311&lang=ja
E-mail: matsuitks[at]stf.teu.ac.jp

■School of Bioscience and Biotechnology Website:
https://www.teu.ac.jp/gakubu/bionics/index.html